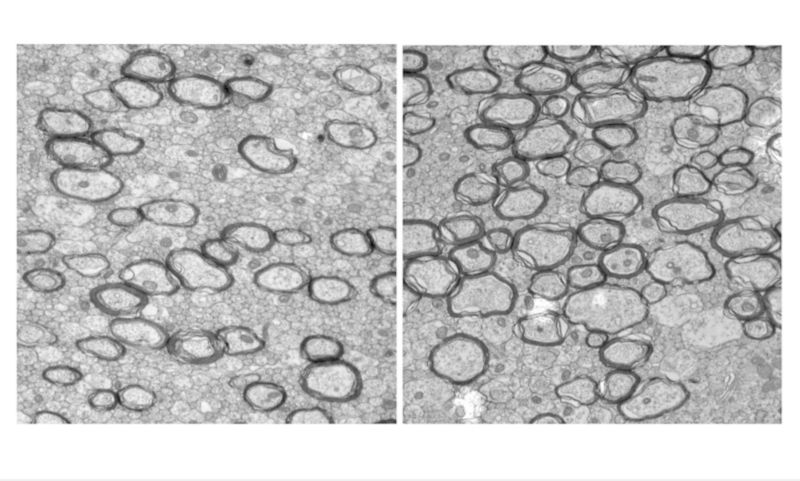
Through a series of clinical and pre-clinical studies, an international research team has for the first time worked out how to calm down hyperactive microglia, the brain’s immune cells.
The research points to a potential treatment for reducing brain injury in premature babies, showing microglia could be successfully targeted with drugs to control their harmful overactive behaviour.
The research, conducted over seven years by scientists and clinicians from Australia, France, the United Kingdom, Germany, Singapore and Sweden, is published in the journal Brain.
About 15 million babies are born prematurely around the world each year and rates of preterm birth are increasing in developed countries. In Australia, around 8% of babies are born prematurely (before 37 weeks gestation).
Premature birth is the most common cause of death and disability in children under 5 and up to 60% of babies born too early will have lifelong problems including attention-deficit disorders, autism, cerebral palsy and epilepsy.
Senior co-author, RMIT University’s Dr Bobbi Fleiss, said previous research had shown that exposure to inflammation was a driver of both premature birth and brain injury in babies.
“Inflammation is our body’s natural way of fighting infection, but the immune cells that drive this response can react too strongly in the developing brain and go into hyperdrive,” Fleiss, a Vice-Chancellor’s Research Fellow at RMIT, said.
“This intense immune response not only damages the baby’s brain, it diverts those cells from their other job of supervising brain development.
“We’ve discovered a way to tone down the hyperactivity without affecting the critical brain building work, using a microglia targeted therapeutic approach.
“It’s incredibly exciting because until now, we’ve known so little about the mechanisms that control the behaviour of microglia.
“This discovery gives us a solid way forward for developing new brain-protective therapies that will help millions of premature babies, and their families, around the world.”