Smart spongy device captures water from thin air
Engineers from Australia and China have invented a sponge-like device that captures water from thin air and then releases it in a cup using the sun’s energy, even in low humidity where other technologies such as fog harvesting and radiative cooling have struggled.
Harnessing data to drive liveability
RMIT is collaborating with government and industry partners on an innovative data sharing project, which aims to create more sustainable, liveable and healthier communities.
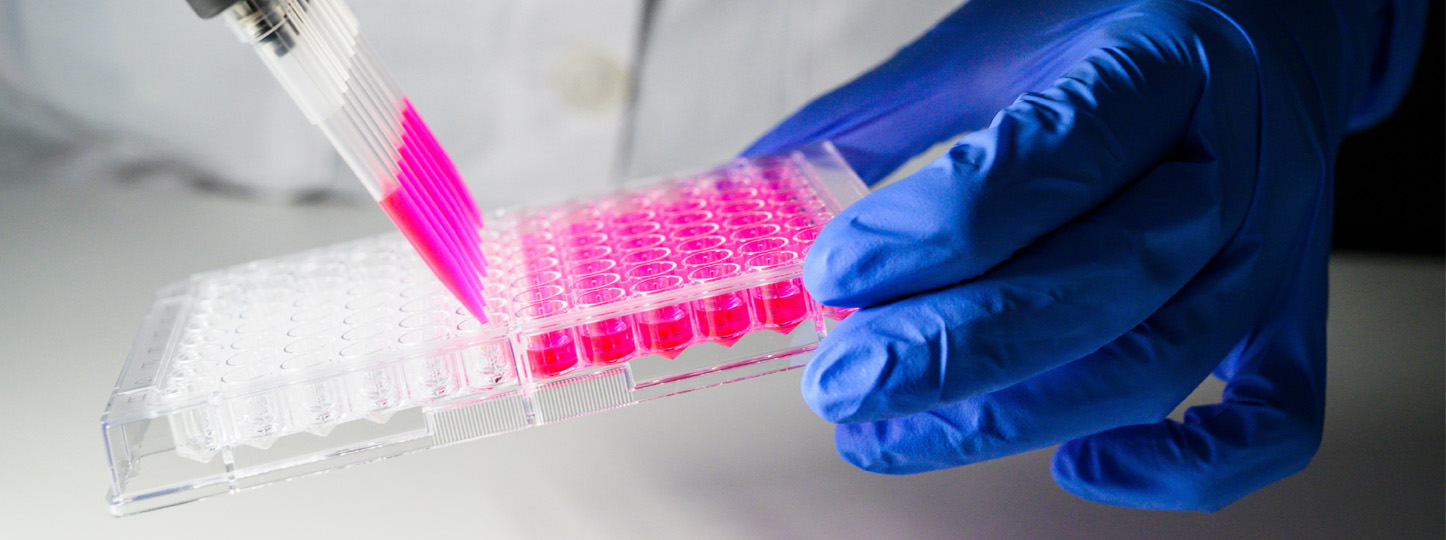
Accelerating health workforce skills through a successful pilot program
Following a successful pilot run by RMIT University’s Health Transformation Lab and College of Vocational Education addressing digital skills shortages, the Victorian Government has increased its support with an additional $4.75 million announced to expand Skills Solutions Partnerships – a platform for training providers and industry to collaboratively develop programs addressing emerging skills needs across Victoria.
Water expert joins RMIT Europe
Drawing on extensive expertise in water resource science from Melbourne, Australia, RMIT’s Professor Vincent Pettigrove is set to collaborate with RMIT Europe’s staff and industry partners over the next six months, particularly through his involvement in a research project aimed at improving coastal resilience throughout Europe.