Scientists call for targeted fibre diets to boost health
Australian food scientists have reclassified dietary fibres – beyond just soluble and insoluble – to better guide nutritional decisions and drive targeted health food products.
More women in tech could deliver a $6.5bn benefit to Australian businesses
A new report by RMIT Online and Deloitte Access Economics has found that increasing women’s participation in technology careers could represent a $6.5 billion opportunity for Australian businesses.
RMIT spin-off launches new manufacturing hub in the heart of Melbourne
The company producing an early fault detection (EFD) system that helps prevent bushfires and blackouts globally has established a new manufacturing hub in Richmond.
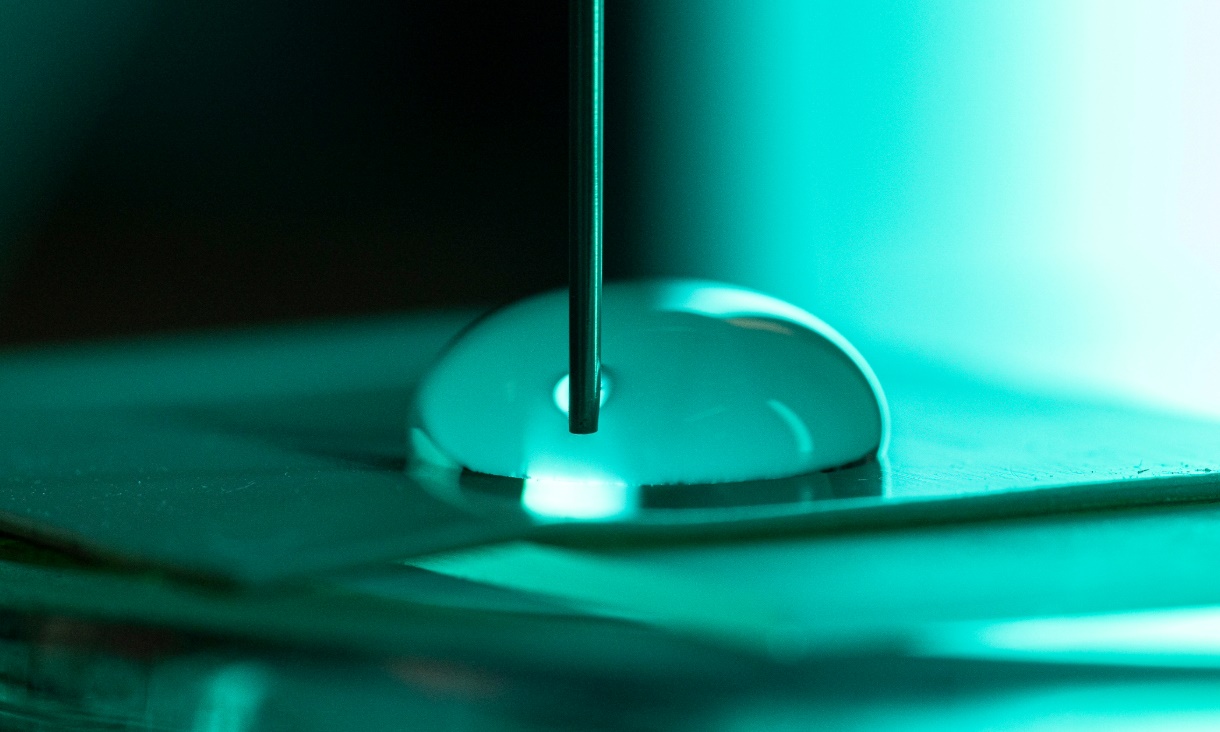
Water movement on surfaces makes more electric charge than expected
Researchers from RMIT University and the University of Melbourne have discovered that water generates an electrical charge up to 10 times greater than previously understood when it moves across a surface.